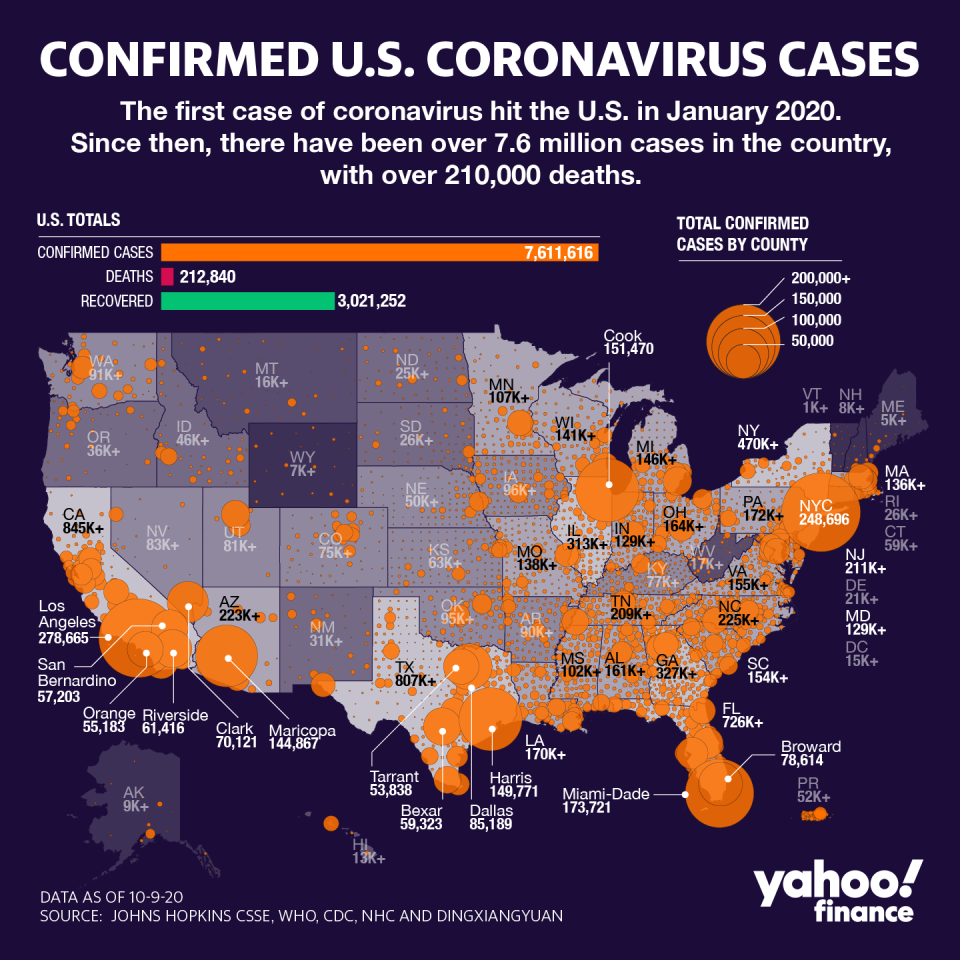
Coronavirus update: J&J and Eli Lilly trials hit pause as US doubles down on antibody therapies
Johnson & Johnson (JNJ) announced late Monday it was pausing shots in its late-stage coronavirus vaccine trial due to an adverse event, but plans to continue enrolling patients and stick to the current manufacturing timeline.
It is still unknown if the participant was receiving a placebo or the vaccine, a point that will be determined by an independent advisory group, known as the Data Safety Monitoring Board (DSMB). Another vaccine using similar technology from AstraZeneca (AZN) is still on hold after a serious adverse event from a trial participant triggered a halt in September. The trial has continued in the U.K., but remains on hold in the U.S.
“Adverse events – illnesses, accidents, etc. - even those that are serious, are an expected part of any clinical study, especially large studies,” J&J said statement late Monday, reiterating that “studies may be paused if an unexpected serious adverse event (SAE)” occurs that may or may not be related to the trials. The company promised a “careful review of all of the medical information before deciding whether to restart the study.”
Mathai Mammen, head of research and development at Janssen, J&J’s pharmaceutical arm, said the information is kept confidential from the company until the DSMB reviews it.
“It will be a few days at minimum for the right set of information to be gathered and evaluated,” Mammen said during an investor call Tuesday.
The two frontrunner candidates in the U.S., Moderna (MRNA) and Pfizer (PFE) with BioNTech (BNTX), are using technology that has never been approved, but have so far not hit any significant adverse events.
Meanwhilel, Pfizer’s non-peer-reviewed data showed some side effects, but nothing that would trigger a halt. Pain at the point of injection and fatigue are considered normal vaccine side effects — seen frequently after flu shots.
Monoclonal antibodies
The experimental treatment used by President Donald Trump while he was at Walter Reed Military Medical Center is gaining momentum as the country awaits a vaccine by the end of the year.
Monoclonal antibody treatments, which are lab-produced antibodies from a sample of recovered patients, has been seen as a bridge between standard treatments and a vaccine, as well as an alternative for those who cannot be given a vaccine. That is because it can both treat and defend against the virus.
Trump received a high dose of Regeneron’s (REGN) antibody cocktail, which sparked the company and Eli Lilly (LLY), which is also developing an antibody treatment, to apply for emergency use authorizations (EUA).
Eli Lilly also paused its antibody trial Tuesday, notifying trial sites that there was a reason for concern. Further details are not yet available.
In response to a request for comment, Lilly told Yahoo Finance the independent safety board (DSMB) has recommended a pause in enrollment.
“The trial, evaluating Lilly’s investigational neutralizing antibody as a treatment for COVID-19 in hospitalized patients, is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH). Lilly is supportive of the decision by the independent DSMB to cautiously ensure the safety of the patients participating in this study,” according to the statement.
The U.S. Food and Drug Administration (FDA) has yet to provide an update on these filings from last week, but analysts have anticipated authorizations following Trump’s treatment.
Meanwhile, Operation Warp Speed announced a collaboration with AstraZeneca to test and produce a monoclonal antibody cocktail, which would be provided for free once authorized by the FDA.
Adding to the portfolio of candidates, the National Institutes of Health (NIH) announced Tuesday it would begin a randomized placebo-controlled trial — the gold standard— to compare investigational antibody therapies.
“The goal here is to identify as quickly as possible the experimental therapeutics that demonstrate the most clinical promise as COVID-19 treatments and move them into larger-scale testing,” said National Institute of Allergy and Infectious Diseases director Anthony Fauci.
He added that the study in 100 participants is “both an efficient way of finding those promising treatments and eliminating those that are not.”
The trial will test a monoclonal antibody developed by Boehringer Ingelheim and AbbVie (ABBV), in conjunction with Gilead Sciences’ (GILD) antiviral drug remdesivir. The latter is the only authorized branded treatment to-date, and has been used as standard protocol for COVID-19 patients, including the president. Recent data showed it worked best on patients who need supplemental oxygen.
More from Anjalee:
Redfield: CDC 'preparing earnestly' for vaccine in November, December
India's tech giants navigate 'literally spiking' WFH demand, H1-B fears in coronavirus era
How protests spurred Corporate America into action on race, inequality
Read the latest financial and business news from Yahoo Finance
Follow Yahoo Finance on Twitter, Facebook, Instagram, Flipboard, SmartNews, LinkedIn, YouTube.
