Coronavirus update: Novartis enters vaccine race as reopenings, testing efforts ramp up
Pharmaceutical companies on Thursday continued their pursuit of a breakthrough in the fight against the coronavirus pandemic, which set a grim new milestone even as states and cities forge ahead with plans to relax the lockdowns that are crippling the U.S. economy.
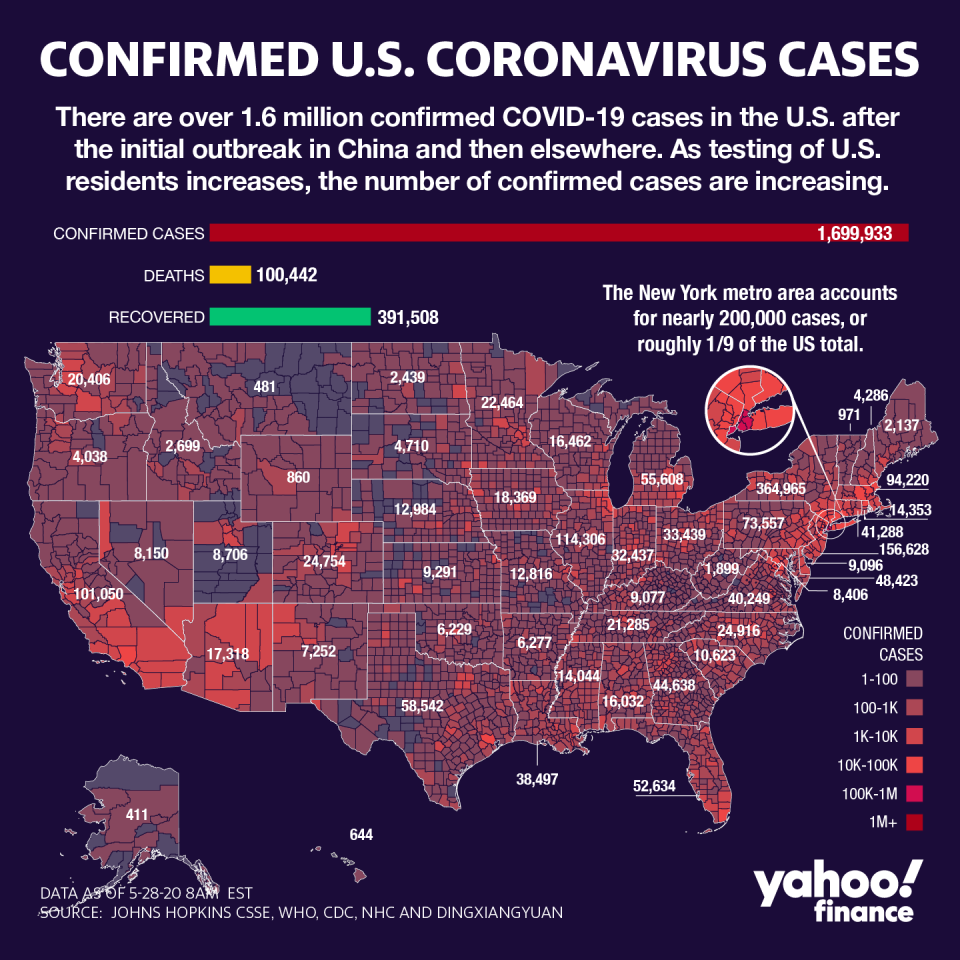
The global COVID-19 case count keeps climbing topping 5.7 million with more than 356,000 dead. The U.S. accounts for nearly a third of those deaths, with diagnoses closing in on 1.7 million nationally on a death toll that topped 100,000 on Wednesday.
The casualties will likely mount until a vaccine is found — which experts say will take at least another year. However, aggressive efforts by some of the biggest names in the pharmaceutical industry have ramped up expectations for a timeline, which have boosted markets in the face of grim economic news.
“As we start to focus on transitioning and coming back online into the workplace, that is giving a sense of optimism to the market,” Philip Drury, Citigroup’s head of EMEA banking and capital markets, told Yahoo Finance on Thursday, but cautioned it was “too soon to call” a rebound.
In a week full of vaccine news, Novartis (NVS) on Thursday announced its entrance into the race for a COVID-19 cure Thursday. In partnership with Harvard Medical School, Mass General Hospital and Massachusetts Eye and Ear, Novartis’ AveXis subsidiary will manufacture a vaccine for COVID-19 using its existing technology at no cost.
AveXis can produce recombinant vaccines, similar to technology being used by Merck (MRK) and Novavax (NVAX), and is providing its manufacturing capacity to the Harvard-led effort, according to the company’s statement. Clinical trials are scheduled to begin in the second half of 2020.
“Having developed and produced one of just two, FDA-approved AAV gene therapies, we are uniquely poised to help the team move quickly toward this accelerated effort,” said Dave Lennon, president of AveXis.
Novartis’ role in the efforts to fight the virus also include its production of hydroxychloroquine, the drug touted by President Donald Trump, but the efficacy of which is doubted by top medical experts and National Institute of Allergy and Infectious Diseases director Anthony Fauci. The World Health Organization and the Food and Drug Administration have also flagged risks associated with using the drug as a COVID treatment.
US turns the corner on testing
Nationally, testing struggles have largely eased as individual states gradually relax restrictions on public lives, and new COVID cases flatten. Large academic and commercial labs are seeing more supply than demand, underscoring how more individuals are getting tested.
CVS Health (CVS) announced it will meet its goal of opening 1,000 drive-up sites at existing store locations around the country Thursday. At least half will serve vulnerable and underserved communities, according to a statement from the company.
CVS began with a limited testing site in mid-March in Massachusetts, followed by more widespread availability in five states. The company previously told Yahoo Finance the strategy relies on the drive-thru pharmacy locations when possible, which preserves personal protective equipment use. Patients will self-swab and CVS will process the samples through partner labs.
Quest Diagnostics (DGX), which has already been using self-swabbing in partnership with Walmart (WMT) for it’s testing sites, received emergency use authorization Thursday to allow individuals to self-swab at home.
This marks a significant step in widespread availability of testing for individuals who do not have access to transportation or are not near a testing site.
"We plan to utilize this device with a range of populations, from state-run programs and employers to healthcare providers and individuals," said Jay Wohlgemuth, Senior Vice President and Chief Medical Officer. "Our scientists at our advanced diagnostics laboratory in San Juan Capistrano, California developed the technology, which has been validated in real-world studies."
The test can be used on adults and children, and can be sent overnight through FedEx’s regular mail — rather than needing an ice pack— to then be tested at a lab, Quest said Thursday.
LabCorp (LH) also previously announced a similar test, but it was limited to health care and other frontline workers only.
Tests are seen as a key ingredient to restarting economic activity, and getting people back to work. In the latest week, an additional 2.1 million individuals filed for unemployment, putting the total numbers out of work at over 40 million. However, continuing claims fell for the first time in weeks, and could be a sign of a businesses reopening.
Separately, the Centers for Disease Control released guidelines for re-opening offices, in an effort to “create a safe and healthy workplace and protect workers and clients.”
Anjalee Khemlani is a reporter at Yahoo Finance. Follow her on Twitter: @AnjKhem
Follow Yahoo Finance on Twitter, Facebook, Instagram, Flipboard, LinkedIn, and reddit.
Find live stock market quotes and the latest business and finance news.
