Coronavirus update: Reopenings continue as virus counts spike in key states; J&J ramps up vaccine push
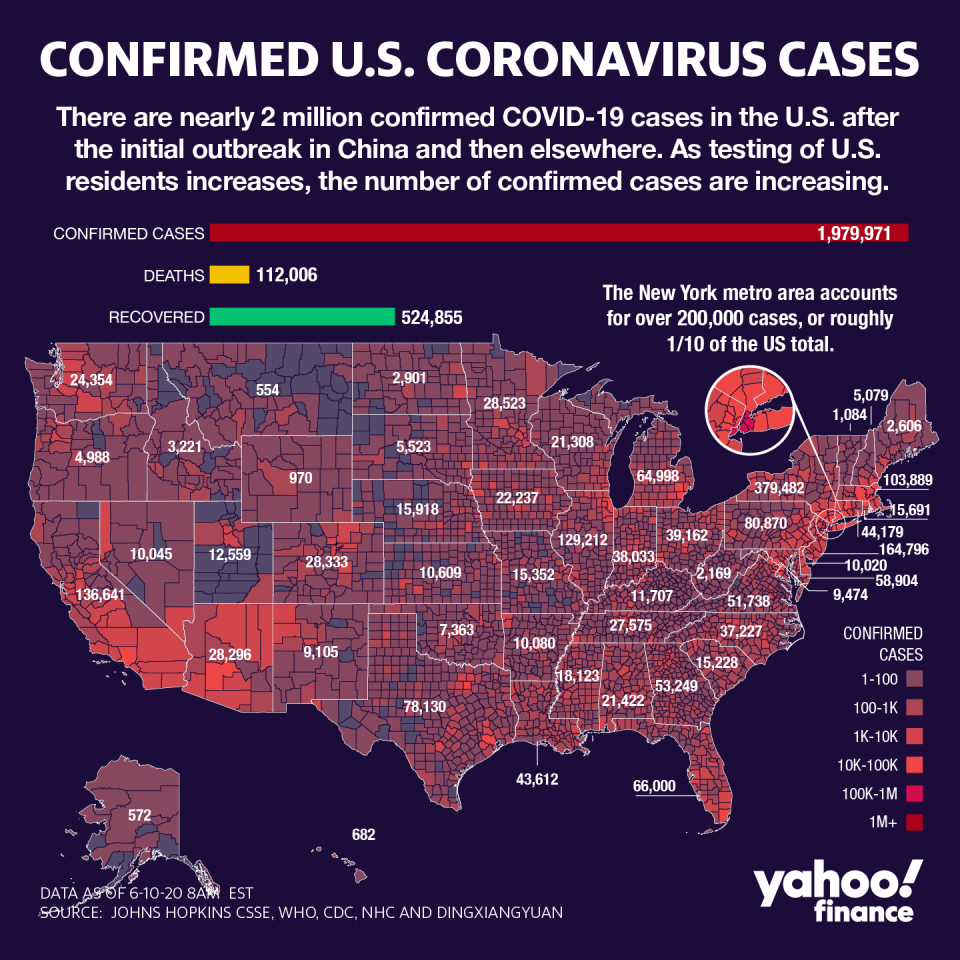
A jump in the coronavirus counts of California and southern states fanned fears of a second wave, even with infections on the decline in the northeast, and other regions forging ahead with their own plans to reopen.
New Jersey and New York City, two major COVID-19 epicenters, lifted stay-at-home restrictions this week as their number of daily cases continues to ebb.
However, public health officials nervously eyed states like Arizona, Florida, North Carolina and Texas, all of which have reported consistent increases in cases. Meanwhile, the virus continues to spread globally, with over 7 million infected worldwide and over 400,000 dead.
Lauren Sauer, assistant professor at Johns Hopkins University, told Yahoo Finance’s On The Move Wednesday that many states have significant cases in rural areas.
“Those are the places where we are likely to see challenges in managing these new surges,” Sauer said. However, since the surge is hitting them later than it originally did the northeast, they may be better-equipped.
Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, and a member of the White House coronavirus task force, told a virtual biotech conference that the novel coronavirus has “devastated the whole world” in a short period of time. “And it isn’t over yet.”
On Tuesday, he told Yahoo Finance that he continues to make calls to gauge the extent of the outbreak’s toll in key areas.
“I screen a lot. In addition to the visible things you see me (do), usually late at night I’m calling up people in different cities and different counties, and trying to find out how things are going,” Fauci said.
“Now, more and more, we’re seeing that the kinds of things that were problems before are being solved,” the doctor added.
Vaccine timeline speeds up
With a lot riding on the path toward finding an effective treatment, Johnson & Johnson (JNJ) announced it is speeding up its vaccine timeline, entering into Phase 1 trials by July rather than September, as projected earlier.
“Based on the strength of the preclinical data we have seen so far and interactions with the regulatory authorities, we have been able to further accelerate the clinical development of our investigational (vaccine). Simultaneously, we are continuing our efforts to build important global partnerships and invest in our vaccine production technology and manufacturing capabilities,” chief scientific officer Paul Stoffels said in a statement.
On Tuesday, Stoffels spoke at the Biotechnology Innovation Organization annual conference about the challenge of ramping up large-scale trials.
He said it was “a Herculean task" to test in "a placebo controlled way with good efficacy points and using good labs.”
The announcement Wednesday came just as reports revealed J&J would receive funding from the National Institute of Health, along with Moderna (MRNA) and AstraZeneca (AZN), for clinical trials and vaccine production.
Anjalee Khemlani is a reporter at Yahoo Finance. Follow her on Twitter: @AnjKhem
Read the latest financial and business news from Yahoo Finance
Follow Yahoo Finance on Twitter, Facebook, Instagram, Flipboard, SmartNews, LinkedIn, YouTube, and reddit.
