Fauci: Sped-up production on coronavirus vaccine risky and costly — but worth it
The government is stepping up its efforts to fund vaccine studies and production for at least three of five frontrunners — something that Dr. Anthony Fauci told Yahoo Finance will be worth its weight in gold in the long run.
In an interview on Tuesday, the nations’s top infectious disease expert said that the risk the government is taking will pay off in terms of time saved when compared to traditional research and development.
“If it isn’t effective and isn’t safe, then the only thing that would have been lost, is money, but we feel it’s worth it to gain those extra months,” said Fauci, the director of National Institute of Allergy and Infectious Diseases.
Typically, the process of drug development waits for each trial phase to complete in order to invest in the next. But the unprecedented global demand for a vaccine as the coronavirus continues to infect millions around the world has pressured everyone into pursuing a cure via a compressed timeline.
At the annual conference of the Biotechnology Innovation Organization (BIO) on Tuesday, Fauci emphasized the need for government funding and not pressuring companies to commit to pricing controls.
“The one thing that is clear is that if you try to enforce things on a company that has multiple different opportunities to do different things, they will walk away,” he said.
Amid concerns that COVID-19 treatments under development might be priced out of reach, Fauci suggested that forcing fair pricing is not as effective as working with companies to ensure a “good faith” effort to provide the vaccine to vulnerable groups and low-income nations.
“Obviously it’s a profit-driven industry. I’ve never seen a successful attempt at doing controls,” Fauci said.
Some of the largest companies are included among the five the government has narrowed down to support as part of Operation Warp Speed. The three receiving trial funding are Moderna (MRNA) — which is furthest along in trials — AstraZeneca (AZN), which is producing Oxford University’s vaccine; and Johnson & Johnson (JNJ), which announced Wednesday it has sped up its timeline to enter trials in July rather than September.
The timeline Fauci outlined includes starting the third and final phase of clinical trials in July, with results expected early in the fall. That may result in a vaccine ready for use — first for the most vulnerable of patients, and frontline and essential workers— by the end of the calendar year.
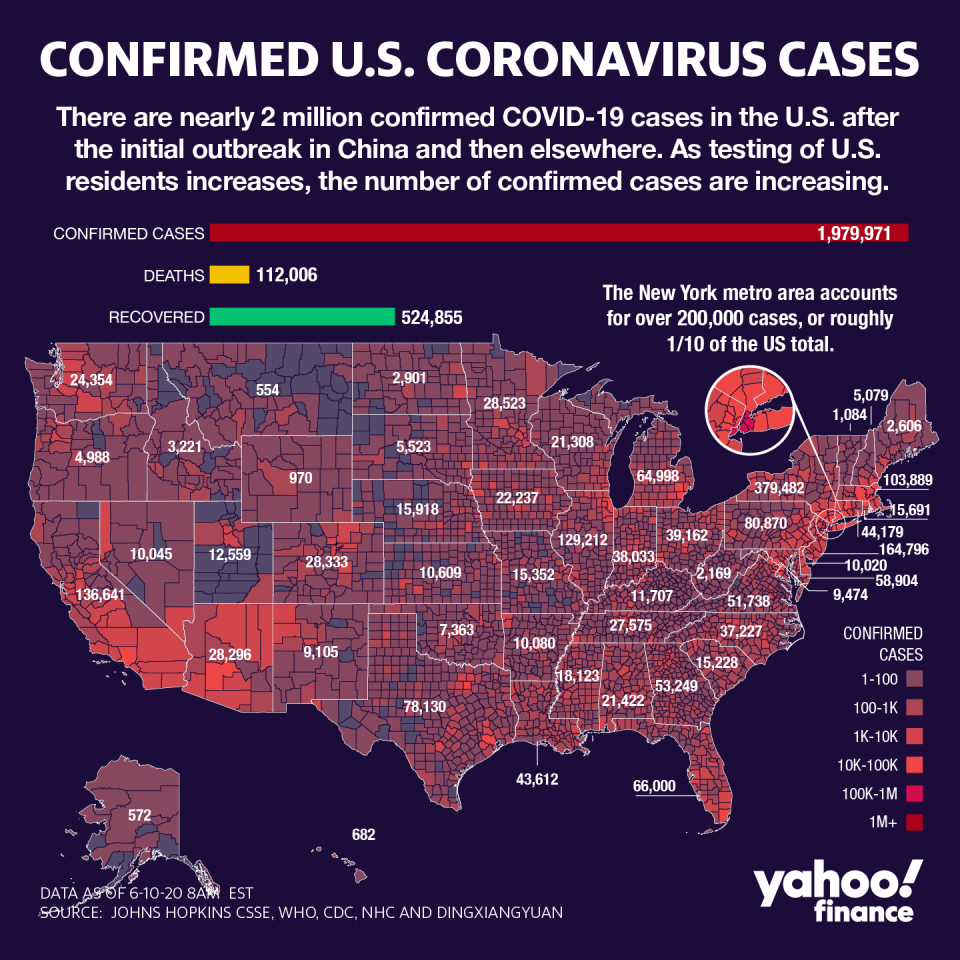
‘Even if you yell, they don’t grow faster’
Funding clinical trials and production of a vaccine is not a new feat for the federal government. It’s how the government responded to the 2009 flu pandemic.
In 2009, Congress appropriated $6.15 billion for the response, including speeding up production of vaccine, on which the government then spent $2 billion on 250 million doses.
At the time, a frustrated Tom Frieden — then director of the Centers for Disease Control — explained why the vaccine still hadn’t reached everyone in the U.S. It was due to the slow pace of traditional vaccine technology, which needs a 6-9 month lead time to grow the virus for use in the vaccines.
“Manufacturers are working hard to get as much vaccine out safely as possible, Frieden said. “The vaccine strains of the virus grow and that's how we develop vaccine. Even if you yell at them, they don't grow faster.”
But the difference was the flu vaccine had a starting point: The flu was a known entity. The coronavirus affecting more than 7.2 million globally, and which has killed more than 422,000, is an entirely novel pathogen that’s forced scientists to make discoveries in real time.
Now, newer technology being used by Moderna, Pfizer (PFE) and others is allowing a quicker timeline to be achieved. Yet Fauci cautioned that won’t guarantee a “safe and effective” cure, he told Yahoo Finance, even though he’s cautiously optimistic.
Anjalee Khemlani is a reporter at Yahoo Finance. Follow her on Twitter: @AnjKhem
More from Anjalee:
Fauci: WHO 'imperfect but important' as coronavirus controversies batter agency
FL teacher explains why she retired because of coronavirus, doubts safe return to schools
How protests spurred Corporate America into action on race, inequality
Read the latest financial and business news from Yahoo Finance
Follow Yahoo Finance on Twitter, Facebook, Instagram, Flipboard, SmartNews, LinkedIn, YouTube, and reddit.
