FDA Rejects Rocket's (RCKT) Gene Therapy BLA for Rare Disease
Rocket Pharmaceuticals RCKT announced that the FDA issued a complete response letter (CRL) to its biologics license application (BLA) seeking approval for its gene therapy Kresladi (marnetegragene autotemcel) to treat patients with severe leukocyte adhesion deficiency-I (LAD-I), a rare genetic disorder.
Per the CRL, the FDA sought ‘limited’ additional information on the therapy’s Chemistry Manufacturing and Controls (CMC), which is a part of the BLA submission. The goal of CMC is to ensure that every batch of the final product is identical in terms of its safety, purity, potency and performance.
Rocket claims that it has already met with the agency officials to align on the scope of the data requested so it can move quickly toward a potential approval.
This is the second time that the FDA requested additional information from the company on Kresladi’s CMC. Earlier this February, the FDA extended the review period of the Kresladi BLA by an additional three months to June 2024 to review the CMC information submitted by Rocket then upon the agency’s request.
The BLA filing is based on data from a phase I/II study, which showed that treatment with Kresladi achieved 100% overall survival for all LAD-I patients at 12 months post-infusion, thereby meeting all primary and secondary endpoints of the study.
Year to date, Rocket’s shares have lost 28.2% compared with the industry’s 6.9% fall.
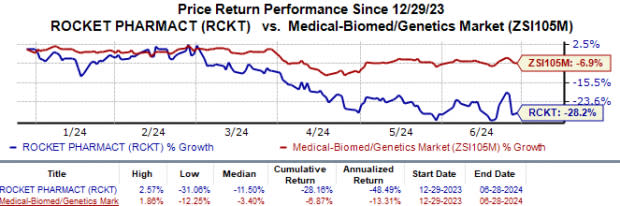
Image Source: Zacks Investment Research
LAD-I is a rare, severe pediatric genetic disorder that is caused by a defect in the ITGB2 gene. This leads to a deficiency in CD18 and can cause recurrent and life-threatening infections, fatally affecting the immune system.
A potential approval to Kresladi represents a significant opportunity for Rocket Pharmaceuticals, which is currently devoid of marketed products. The only curative treatment for LAD-I is an allogeneic bone marrow transplant, which is often not readily available and carries substantial morbidity and mortality risk. If approved, Kresladi could help provide patients with a less risky and more easily available treatment option.
Apart from Kresladi, Rocket is also evaluating other hematological candidates in its pipeline. This includes RP-L102, which is currently being reviewed by the EMA as a potential treatment for patients with Fanconi anemia (FA). This filing is supported by data from a mid-stage study, which showed that FA patients treated with RP-L102 achieved sustained genetic corrections and comprehensive phenotypic correction at 12 months post-infusion.
Though management did announce its intent to submit a similar regulatory filing for RP-L102 in FA indication with the FDA in first-half 2024, it is yet to make any announcements regarding the same.
Rocket is also evaluating a third gene therapy candidate in hematology, namely RP-L301. Management is in the process of starting a pivotal mid-stage study on RP-L301 in patients with pyruvate kinase deficiency (PKD).
Rocket is also focused on developing gene therapies targeting cardiovascular (CV) diseases. Management is developing adeno-associated virus (AAV) gene therapies to target mutations or defects in different genes. The most advanced AAV-based candidate in the company’s pipeline is RP-A501, which is in a mid-stage study to treat Danon disease (DD).
Another AAV-based gene therapy candidate is RP-A601, which is in early-stage development for arrhythmogenic cardiomyopathy (ACM). Rocket also plans to start clinical studies on a new gene therapy candidate for treating dilated cardiomyopathy (DCM) later this year.
Zacks Rank & Key Picks
Rocket currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the overall healthcare sector include Arcutis Biotherapeutics ARQT, Compugen CGEN and Heron Therapeutics HRTX, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 60 days, estimates for Arcutis Biotherapeutics’ 2024 loss per share have narrowed from $2.22 to $1.60. During the same period, the loss estimates per share for 2025 have narrowed from $1.37 to $1.14. Year to date, shares of Arcutis have surged 187.9%.
Earnings of Arcutis Biotherapeutics beat estimates in three of the last four quarters while missing the mark on one occasion. Arcutis delivered a four-quarter average earnings surprise of 14.93%.
In the past 60 days, estimates for Compugen’s 2024 earnings per sharehave risen from 2 cents to 5 cents. During the same period, loss estimates per share for 2025 have improved from 27 cents to 11 cents. Year to date, CGEN’s shares have lost 15.2%.
Earnings of Compugen beat estimates in three of the last four quarters while missing the mark on one occasion. CGEN delivered a four-quarter average earnings surprise of 5.79%.
In the past 60 days, estimates for Heron Therapeutics’ 2024 loss per sharehave narrowed from 24 cents to 10 cents. During the same period, estimates for 2025 have improved from a loss of 8 cents to earnings of 1 cent. Year to date, HRTX’s shares have appreciated 105.9%.
Earnings of Heron Therapeutics beat estimates in three of the last four quarters while missing the mark on one occasion. HRTX delivered a four-quarter average earnings surprise of 30.33%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Compugen Ltd. (CGEN) : Free Stock Analysis Report
Heron Therapeutics, Inc. (HRTX) : Free Stock Analysis Report
Rocket Pharmaceuticals, Inc. (RCKT) : Free Stock Analysis Report
Arcutis Biotherapeutics, Inc. (ARQT) : Free Stock Analysis Report