How Gilead's coronavirus drug breakthrough is setting the tone for other potential treatments
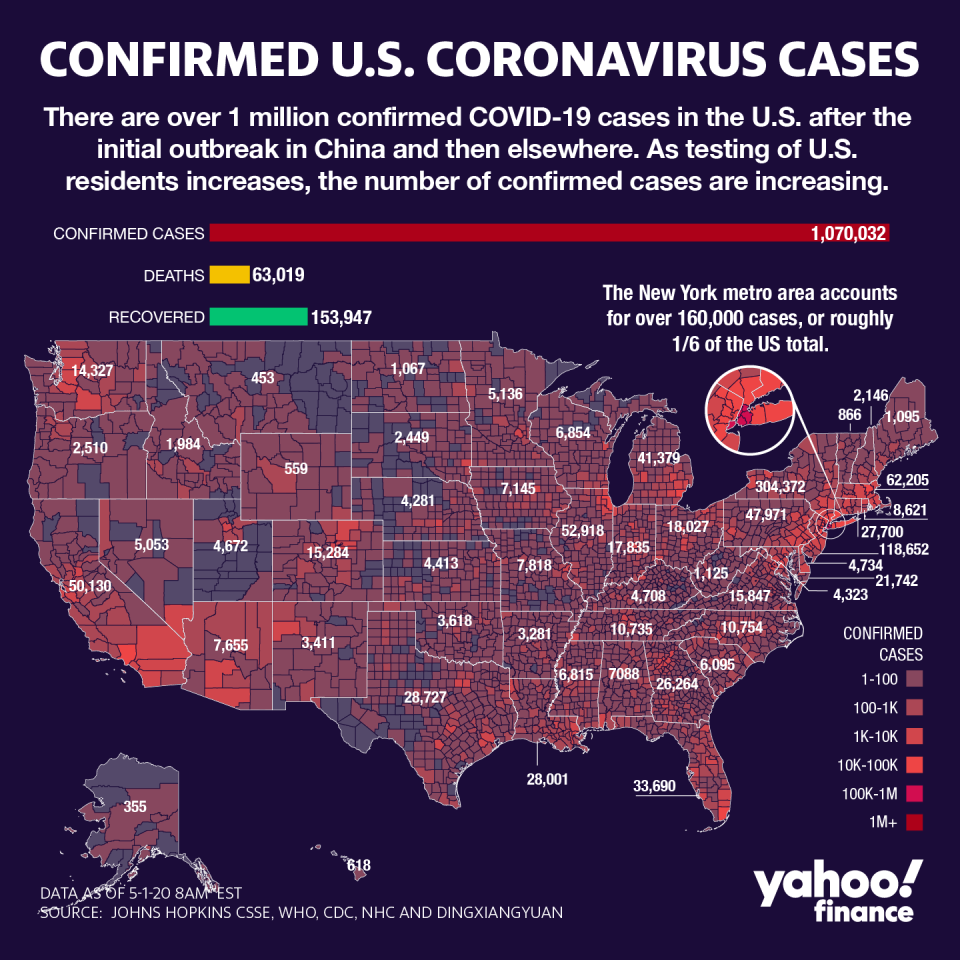
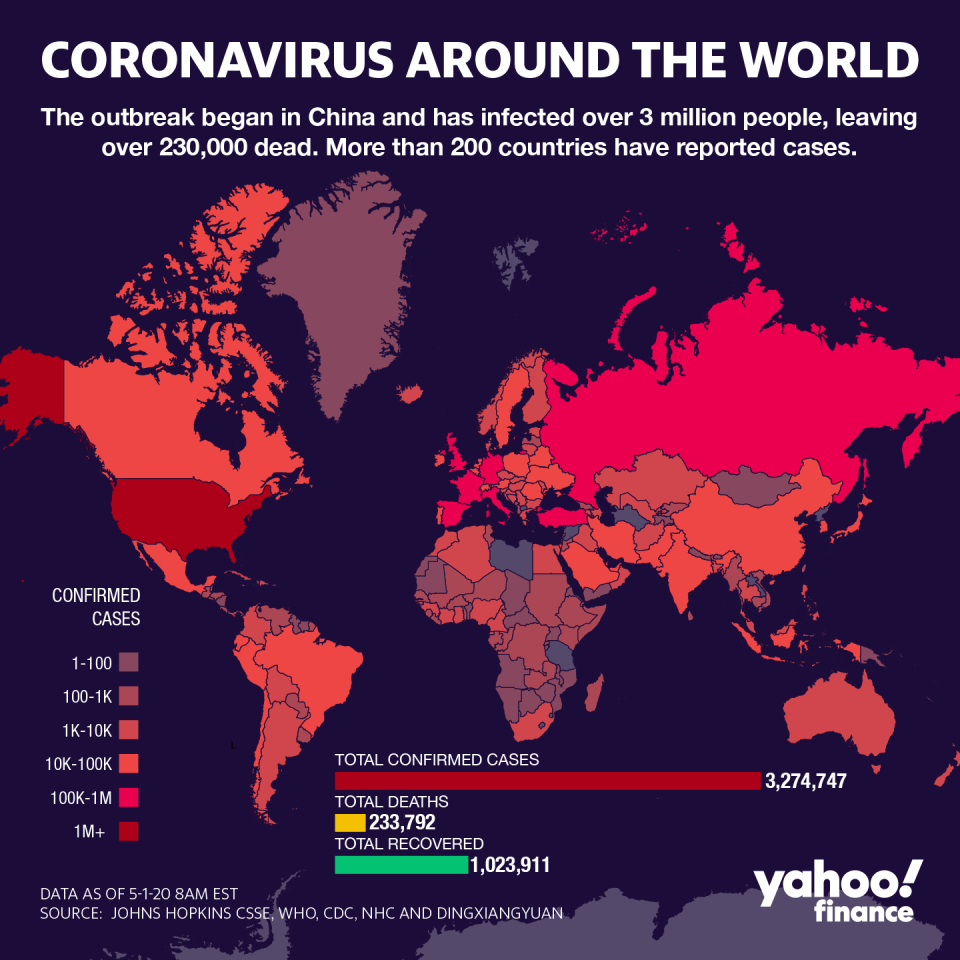
Gilead Sciences (GILD)’s clinical trial results for a potential coronavirus treatment are being heralded as a breakthrough, largely by helping to blaze a trail for other drugs in the pipeline, while illuminating a path forward in the fight against a “scary” pandemic that’s infected over 3 million around the world.
Three separate studies of Gilead’s remdesivir — including a placebo trial by the National Institute of Allergy and Infectious Disease, which carries far more influence — have helped backstop markets, and sparked hopes that the COVID-19 crisis can be managed.
The encouraging results have also opened the door for Gilead to pursue an emergency use authorization from the U.S. Food and Drug Administration, which was approved Friday.
The FDA said the drug can now be used to treat “suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease. Severe disease is defined as patients with low blood oxygen levels or needing oxygen therapy or more intensive breathing support such as a mechanical ventilator.”
The timeline from trials to approval for the experimental drug is significant, according to Dr. Deborah Birx, coordinator of the White House coronavirus task force.
“I think this really illustrates what can happen in such a short time. For the first case, that was diagnosed in the United States to now, our first step forward with a therapeutic in less than 90 days,” she said.
Gilead CEO Daniel O’Day said the company is pushing for full approval of the drug.
“There’s a big sense of urgency here, I think the FDA understands the importance of reacting quickly to this,” CEO Daniel O’Day said on an earnings call Thursday. “It’s intense right now and...we think the FDA will move quite quickly on their decision on the labeling side.”
He added he believes the regulatory authority will first approve the drug for emergency use, followed by full approval. But even with emergency use, the company could start collecting revenues on the drug, even though it hasn’t determined a price for it yet.
The drug is currently only available in intravenous form, but the company is exploring other options including an inhaler. Company executives said an oral form was not an option because of hazards to the liver.
However, the CEO underscored that earning profits is not the goal just yet, as the company plans to donate its entire current supply, some 1.5 million doses, to clinical trials in the U.S. and around the world. Patients may also obtain the drug via existing compassionate use authorization.
Gilead also plans to produce enough doses to treat 1 million patients by the end of the year, and is working to expand its supply chain with larger industry partners — but it has to do so without jeopardizing its access to limited raw materials for the drug, O’Day said.
NIAID’s director, Dr. Anthony Fauci, said Wednesday that while the drug is not a “knockout,” the agency’s study shows the virus does respond to drugs, which opens the door to more treatment options.
“The data shows that remdesivir has a clear-cut, significant, positive effect in diminishing the time to recover...what it has proven is that a drug can block this virus,” Fauci said, even as he cautioned the data needed more rigorous, statistically significant analysis.
Former FDA official Henry Miller cautioned while the news was positive, “it is only the beginning.”
Miller told Yahoo Finance that he assumes the FDA has reviewed the clinical trial data in detail, but that further studies and real-world clinical experience will provide greater information about which patients benefit the most or least from the drug, and at which stages of the infection.
“There’s far more to a drug approval (or EUA) than just reading an account of the clinical trial,” he said.
A drug’s promise against a ‘scary’ problem
Gilead’s results showed slight improvements in recovery time of severe patients given the drug intravenously. Patients recovered in 11 days with the drug, compared to 15 without, and the mortality rate was 8% for those with the drug, compared to 11% without.
While there’s more work ahead, one thing is clear from the clinical trial results — the COVID-19 drug development landscape now has a clearer path forward. As Gilead’s CEO put it on Thursday, “one now has to compare with remdesivir or work with remdesivir.”
The drug’s encouraging test results have helped bolster investor psychology in a market that’s desperate for good news in the increasingly grim fight against the coronavirus — especially with hopes of ending the lockdown inextricably linked to effective COVID-19 testing and treatment options.
“Because it tells us a drug can work against this terrifying, scary, global problem,” Brad Loncar, a biotech investor and CEO of Loncar Investments, told Yahoo Finance.
Gilead’s study in severe patients showed a different aspect of the effectiveness of the drug. Patients who took a 5-day treatment course saw similar results as those on a 10-day course. Loncar estimates the company now has enough to treat 280,000 people, double the previous estimate, and Gilead plans to invest $1 billion to ramp up manufacturing.
In a statement Friday, Gilead said the current treatment course will remain 10 days.
“The optimal dosing and duration of remdesivir for the treatment of COVID-19 is still unknown. Under this EUA, the 10-day dosing duration is suggested for patients requiring invasive mechanical ventilation...and the 5-day dosing duration is suggested for patients not requiring invasive mechanical ventilation,” Gilead said.
The studies have also provided a clue about how to attack the virus in order to create a tailor-made solution. It suggests pharmaceutical companies can create drug cocktails — like those used to treat HIV patients— with other treatments currently being studied.
For his part, Fauci expressed cautious optimism that an anti-inflammatory drug currently being studied may be used in combination with remdesivir. “So as drugs come in, we’re going to see if we can add on that,” he said.
Both Kevzara, a drug co-developed by Regeneron (REGN) and Sanofi (SNY), and Actemra, a drug developed by Roche (RHHBY), are among those being studied as treatment options.
However, more information is still needed, as the NIH trial heads to a second phase in the summer. Sanofi recently said it amended the Kevzara study to focus on the most critical patients, saying it saw negative results in less-critical patients.
Gilead is also looking at the most critical patients, including those on ventilators, in an expansion of the current trial, and has emphasized that it’s emergency use authorization is not the equivalent an approval.
“It is not yet known if remdesivir is safe and effective for the treatment of COVID-19,” the company said.
Lingering questions
Earlier this week, Trump called the Gilead “a beginning” that provides a good building block for the future.
Meanwhile, the FDA is working to get Gilead’s drug to the patients who need it most. In a statement to Yahoo Finance, an FDA spokesperson said, “the agency has been engaged in sustained and ongoing discussions with Gilead Sciences regarding making remdesivir available to patients as quickly as possible, as appropriate.”
Meanwhile, as that process moves along, an open question remains how politics may play a role. Gilead intends to distribute the drug globally — but the crisis has worsened bilateral ties between the U.S. and China, while putting severe strains on the global supply chain.
“It will be essential for countries to work together to create enough supply for people all over the world and... we are in discussions with various groups about how we might bring remdesivir to the developing world,” Gilead’s O’Day wrote in a recent letter.
However, some observers like Loncar are concerned if the federal government could intervene in a way that hamstrings development.
“If U.S. protectionism and politics gets thrown into this...is Gilead only going to be able to be used only in the U.S., or will Trump use it as a political tool? Like sending it to allies like Italy and France but not sending it to Iran and China,” Loncar asked.
[Click here for more of Yahoo Finance’s coronavirus coverage: Personal finance tips, news, policy, graphics & more from Yahoo Finance]
Anjalee Khemlani is a reporter at Yahoo Finance. Follow her on Twitter: @AnjKhem
Follow Yahoo Finance on Twitter, Facebook, Instagram, Flipboard, LinkedIn, and reddit.
Find live stock market quotes and the latest business and finance news.
