E-cigs are twice as effective as nicotine replacement at helping smokers quit, study shows
Under intensifying scrutiny from the U.S. Food & Drug Administration’s crackdown on the teen vaping epidemic, e-cigarette manufacturers may have finally found some support for their existence.
Juul, the country’s largest e-cigarette company, and its peers have long maintained that their products are first and foremost meant to exist on the market as smoking cessation devices rather than a nicotine hook for teens.
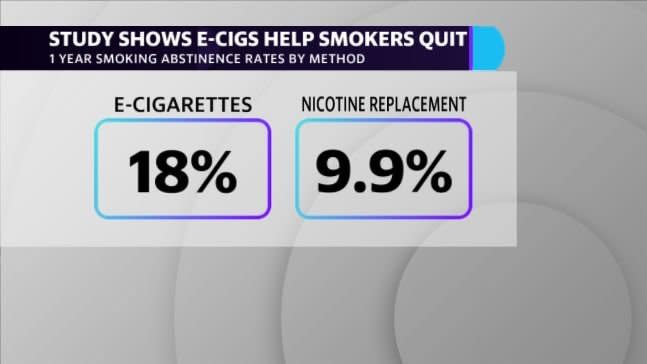
A new study, published Wednesday in the New England Journal of Medicine, was among the first to finally support those claims. In tracking nearly 900 smokers for a year in their quest to abstain from smoking, the independent study found e-cigarettes to be nearly twice as effective at helping smokers quit than traditional nicotine replacement therapies. The one-year abstinence rate for smokers in the e-cigarette group was 18% compared to just 9.9% for those treated with nicotine replacement products like nicotine gum, inhalers or patches.
However, the study did call for more research into the potential downsides that could exist that comes with replacing smoking traditional cigarettes with their electronic alternative.
“This can be seen as problematic if e-cigarette use for a year signals ongoing long-term use, which may pose as-yet-unknown health risks,” the study’s authors wrote. The study did not use Juul e-cigarettes, but rather One Kit brand e-cigarettes which feature much lower concentrations of nicotine.
The benefit of e-cigarettes, advocates note, is their ability to deliver nicotine smokers crave without the negative carcinogens and toxins that are created as a byproduct with the burning of tobacco. E-cigarette companies, however, are not approved in the U.S. to market their products as smoking cessation devices. Philip Morris International is still awaiting the FDA approval to do just that with their heat-not-burn device iQOS.
FDA straddles the e-cig debate
But the FDA and its commissioner Dr. Scott Gottlieb has been straddling the e-cigarette debate. On the one hand, e-cigarettes present an enticing option to help smokers quit and could help prevent the nearly 6 million deaths caused by tobacco use worldwide, according to the Centers for Disease Control and Prevention. On the other, anti-tobacco advocates have derided the FDA for not regulating e-cigarette companies strongly enough as teens increasingly take up vaping.
That epidemic has been reiterated in study after study last year — the latest from the National Institute on Drug Abuse showed a near doubling in the rate of high school seniors using e-cigarettes, jumping from 11% to nearly 21% in just a year. The study also showed more than 1-in-10 middle schoolers reported vaping in the past year, as Juul sales quintupled from $200 million to $1 billion.
In lockstep with changes the FDA wanted to implement, Juul announced last year it would stop selling flavored e-cigs at retail locations and would increase age-verification methods online.
Since then, Gottlieb has stated that the agency could seek stricter regulations if improvements in the teen vaping epidemic are not seen soon. This week, before the New England Journal of Medicine published their findings, Gottlieb alluded on Twitter to more regulations, writing, “The #FDA has taken unprecedented steps to address kids’ use of e-cigs while maintaining access to currently addicted adult smokers. But these points are received. The agency must - and soon will - be doing much more to firmly address this mounting epidemic.”
Whether this latest study’s findings give the FDA pause remains to be seen.
Zack Guzman is the host of YFi PM as well as a senior writer and on-air reporter covering entrepreneurship, startups, and breaking news at Yahoo Finance. Follow him on Twitter @zGuz.
Read more:
Why the vaping craze is an opportunity for Philip Morris
Juul surpasses Facebook as fastest startup to reach decacorn status
How Juul became the FDA’s latest target
Joe Camel illustrator: E-cig maker Juul’s marketing ‘seems more egregious’